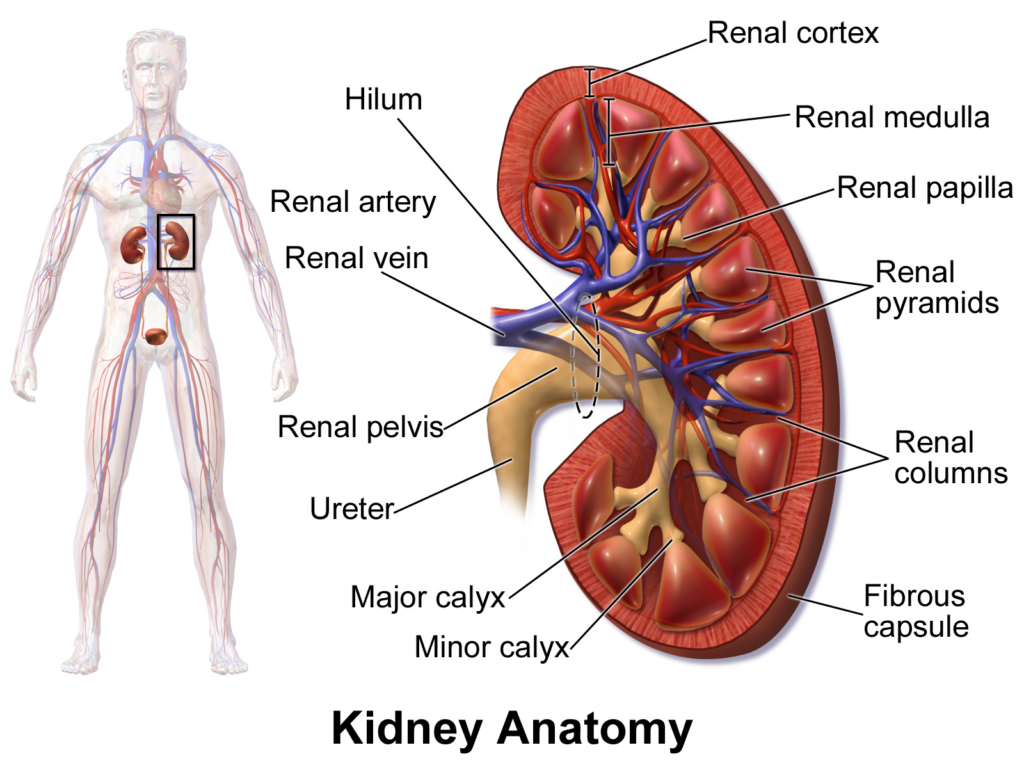
A 62-year-old man with end-stage renal disease has become the first human to receive a new kidney from a genetically modified pig, doctors from Massachusetts General Hospital in Boston announced on Thursday. https://www.reuters.com/business/healthcare-pharmaceuticals/first-pig-to-human-kidney-transplant-performed-boston-2024-03-21/
The four-hour surgery, performed on March 16, “marks a major milestone in the quest to provide more readily available organs to patients,” the hospital said in a statement.
The patient, Richard Slayman of Weymouth, Massachusetts, is recovering well and expected to be discharged soon, the hospital said.
Experts are keenly interested in long-term results of the ground-breaking animal-to-human transplant, said Dr Jim Kim, director of kidney and pancreas transplantation with USC Transplant Institute in Los Angeles.
Slayman had received a transplant of a human kidney at the same hospital in 2018 after seven years on dialysis, but the organ failed after five years and he had resumed dialysis treatments.
The kidney was provided by eGenesis of Cambridge, Massachusetts, from a pig that had been genetically edited to remove genes harmful to a human recipient and add certain human genes to improve compatibility. The company also inactivated viruses inherent to pigs that have the potential to infect humans.
Kidneys from similarly edited pigs raised by eGenesis had successfully been transplanted into monkeys that were kept alive for an average of 176 days, and in one case for more than two years, researchers reported in October in the journal Nature.
Drugs used to help prevent rejection of the pig organ by the patient’s immune system included an experimental antibody called tegoprubart, developed by Eledon Pharmaceuticals (ELDN.O), b.
The surgery marks progress in xenotransplantation – the transplanting of organs or tissues from one species to another – said Dr Robert Montgomery, director of the NYU Langone Transplant Institute, who was not involved in the case.
The field “is marching closer to becoming an alternative source of organs for the many hundreds of thousands suffering from kidney failure,” he said in an email.
According to the United Network for Organ Sharing, more than 100,000 people in the U.S. await an organ for transplant, with kidneys in the greatest demand.
NYU surgeons had previously transplanted pig kidneys into brain-dead people.
Montgomery said transplant centres are taking different approaches in terms of gene edits and medications, adding that “another big step will be when the FDA authorizes clinical trials so we may better understand what will work best for patients on our waiting lists.”
A University of Maryland team in January 2022 transplanted a genetically modified pig heart into a 57-year-old man with terminal heart disease, but he died two months later.
////
President Joe Biden this week provided some details of how he plans to fulfil a pledge in this month’s State of the Union to devote $12 billion in new funding to women’s health research. www.science.org/content/article/news-glance-geoengineering-test-canceled-havana-syndrome-probed-and-u-s-energy-research?
He signed an order directing the National Institutes of Health (NIH) and other research and health agencies to prioritize and coordinate research projects, including some using artificial intelligence, to study topics such as menopause and osteoporosis. Biden also highlighted that his budget request for the 2025 fiscal year includes $200 million for a new fund at NIH to support interdisciplinary research and a network of research centres devoted to women’s health. He provided no details about the remaining $11.8 billion.
////
The first immune-cell therapy for solid tumours, Iovance Biotherapeutics’ lifileucel (Amtagvi), has been approved by the US Food and Drug Administration. At least 20 people with advanced melanoma will receive the treatment, which uses tumour-infiltrating lymphocytes (TILs) extracted from a person’s own tumour. Researchers hope that the approval will pave the way for cheaper versions — lifileucel costs more than half a million dollars — as well as similar therapies for other cancers, including lung and pancreatic tumours.
////
Engineered immune cells known as chimeric antigen receptor T (CAR T) cells show promise against an aggressive and deadly brain cancer in two preliminary studies. In one study, three people’s glioblastoma appeared to shrink after CAR-T therapy but then later recurred. Yet, one person’s response lasted for more than six months. In the other study, tumours appeared to shrink in all six of the people treated. One person’s cancer began to grow again within a month, but another has not shown signs of the tumour growing for seven months. The average length of survival for people with glioblastoma is eight months. The results add to mounting evidence that CAR T cells could be modified to treat a wider range of cancers than only blood cancers.
Nature | 4 min read
////
The antiviral drug obeldesivir might be able to cure Ebola Sudan infections, the second most common cause of Ebola outbreaks. All five monkeys that were given obeldesivir one day after receiving a lethal dose of Sudan ebolavirus survived. If the drug proves to be effective in humans, it would be the first oral treatment for any type of Ebola. In laboratory experiments, the drug was active against all known species of Ebola and Marburg, a related virus. The drug could profoundly change Ebola responses, says physician and epidemiologist Armand Sprecher.
The US has approved the first drug to treat an obesity-linked liver disease that affects an estimated 5% of the world’s adults. Resmetirom, to be marketed as Rezdiffra, treats metabolic dysfunction-associated steatohepatitis (MASH) — formerly known as non-alcoholic steatohepatitis (NASH). After many earlier failures, resmetirom is the first to reduce scar tissue known as fibrosis in the liver. But researchers caution that evidence for long-term benefits is still needed. “Only time will tell,” says gastroenterologist Maya Balakrishnan. “In the end, what matters is: Does this drug improve survival?”
Nature | 4 min read
////
The U.S. Centers for Disease Control and Prevention (CDC) on 18 March issued a health advisory urging people, particularly children and international travellers, to get vaccinated against measles due to the increase in cases this year. www.reuters.com/world/us/us-cdc-urges-measles-vaccinations-amid-rising-cases-2024-03-18/
The CDC had recorded 58 cases of the disease across 17 jurisdictions, as of March 14, same as the whole of 2023.
Most cases reported this year have been among children aged 12 months and older who had not received the measles-mumps-rubella (MMR) vaccine, the CDC said, and asked healthcare providers to ensure children are vaccinated against the disease.
The agency said the risk of widescale spread was low, given the currently high immunity levels in the population against measles in most U.S. communities, but added some pockets may have a greater likelihood of outbreaks.
The American Medical Association has also urged Americans to get vaccinated against measles.
“We are reminding physicians to talk with their patients about the health risks associated with not being vaccinated and to make a strong recommendation for vaccinations, unless medically inadvisable,” said Jesse M. Ehrenfeld, president of the U.S. doctors’ body.
Measles is one of the most contagious human viruses and is almost entirely preventable through vaccination. It requires 95% vaccine coverage to prevent outbreaks among populations.
However, according to the CDC, coverage with measles vaccines among U.S. children in kindergarten has decreased to 93.1% in the 2022–2023 school year from 95.2% in 2019–2020.
This has left approximately 250,000 kindergartners susceptible to the disease each year over the last three years, the CDC added.
Declines in measles vaccination rates globally have also increased the risk of measles outbreaks worldwide. The agency added that cases continue to be brought into the United States by travellers infected in other countries.
////
Lalita Panicker is Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi

